An overview of ELEVIDYS clinical trials
The ELEVIDYS clinical trial program included more than 200 participants across multiple trials.
3 to 20 years old*

Ambulatory and non-ambulatory†
A range of genetic mutations‡
* ELEVIDYS is approved for individuals at least 4 years old with Duchenne.
† Safety information in non-ambulatory people is limited to 8 clinical trial participants.
‡ ELEVIDYS cannot be used in anyone with a deletion in exon 8 and/or exon 9 in the dystrophin gene.
There were 3 main trials
Each trial included different people and had specific goals

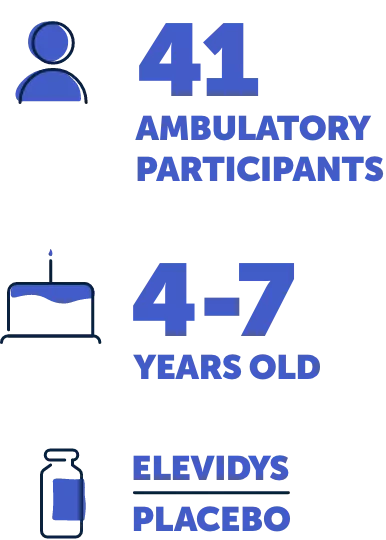
Study 1 was a 2-part, placebo-controlled trial of 41 ambulatory people aged 4 to 7 years. In Part 1, which lasted 48 weeks, the participants were randomly split into 2 groups: 1 that received ELEVIDYS and 1 that received a placebo (a substance with no active medicine).
Part 2 of the trial, which also lasted 48 weeks, reversed the groups, so those who had received ELEVIDYS in Part 1 now received a placebo, and vice versa. No one knew who was in each group.
MAIN GOALS WERE TO MEASURE
- ELEVIDYS micro-dystrophin
- Impact on muscle function
- Safety


Study 2, also called ENDEAVOR, is an open-label trial, which means everyone knew they were receiving ELEVIDYS. This trial included:
- 40 ambulatory participants aged 3 to 12 years
- 8 non-ambulatory participants aged 10 to 20 years
MAIN GOALS WERE TO MEASURE
- ELEVIDYS micro-dystrophin
- Safety
Muscle function was not a main goal of this study.
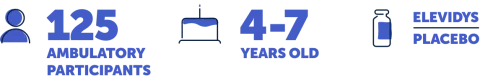
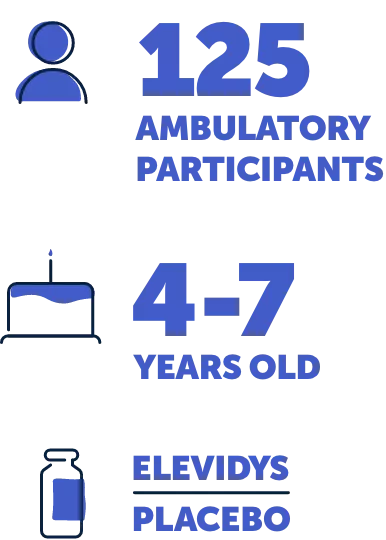
Study 3, also called EMBARK, is a 2-part, placebo-controlled trial of 125 ambulatory people aged 4 to 7 years. In Part 1, which lasted 52 weeks, the participants were randomly split into 2 groups: 1 that received ELEVIDYS and 1 that received a placebo. Part 2 of the trial, which also lasted 52 weeks, reversed the groups, so those who had received ELEVIDYS in Part 1 now received a placebo, and vice versa. No one knew who was in each group. Results from Part 1 are shared on this website.
Main goals WERE TO MEASURE
- Impact on muscle function
- Safety

Results measured

If ELEVIDYS micro-dystrophin was produced in skeletal muscles

If ELEVIDYS micro-dystrophin worked properly, measured through impact on muscle function

Safety, or what side effects people experienced after ELEVIDYS
Multiple trials continue to gather information about the long-term impact of ELEVIDYS.
Two ELEVIDYS trials included a placebo group: Study 1 and Study 3. Participants in the placebo group were treated exactly the same as the ELEVIDYS-treated participants, except they did not receive ELEVIDYS during their infusion. Comparing results from these 2 groups helps researchers understand the impact of treatment. These individuals did have the opportunity to receive ELEVIDYS later in the trial.
Two ELEVIDYS trials included a placebo group: Study 1 and Study 3. Participants in the placebo group were treated exactly the same as the ELEVIDYS-treated participants, except they did not receive ELEVIDYS during their infusion. Comparing results from these 2 groups helps researchers understand the impact of treatment. These individuals did have the opportunity to receive ELEVIDYS later in the trial.

Next up: Learn about eligibility
Now that you’ve explored the clinical trials, find out more about who may be eligible to receive ELEVIDYS.