Understanding eligibility
Understanding eligibility

Who can receive ELEVIDYS
Many families have questions about whether ELEVIDYS is an appropriate treatment. One of the first steps is to consider eligibility. Below are answers to common questions about who may receive ELEVIDYS.
ELEVIDYS is approved for people with Duchenne aged 4 years and up, regardless of ambulation. Use in non-ambulatory people was approved under accelerated approval, which allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit.
Treatment with ELEVIDYS increased the marker, ELEVIDYS micro-dystrophin. There were a limited number of participants who were non-ambulatory (8), and the impact on motor function was not studied in non-ambulatory people.
Verification of clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory people with Duchenne. Studies are ongoing to assess this potential impact.
A doctor at a treatment center that administers ELEVIDYS will confirm eligibility.
All mutations except for deletions in exons 8 and/or 9 are eligible. Note: duplication mutations in exons 8 and/or 9 may be eligible.
People with certain mutation deletions (in exons 1 to 17 and/or exons 59 to 71) may be at risk for an immune response affecting muscles (immune-mediated myositis).
Talk to your doctor about your genetic test results.
A genetic test is required to confirm a mutation in the dystrophin gene. If you’ve had a test already, provide the report to your doctor to see if an updated test may be needed.
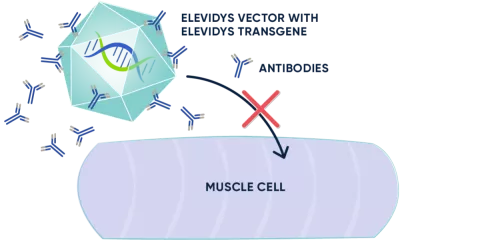
An antibody test is required to measure preexisting antibodies to the ELEVIDYS vector, called AAVrh74. If the levels are too high, ELEVIDYS may not be an option.
Your doctor will perform additional tests to confirm ELEVIDYS is appropriate.
Why antibodies matter
Antibodies are an important part of the immune system. They’re proteins your body creates to protect you from things like viruses. When a virus enters the body, the immune system gets to work, making antibodies to fight it. Once the virus is gone, the antibodies stay in your body, ready to attack that particular virus if it appears again.
VECTOR
Gene therapies, like ELEVIDYS, use vectors as the delivery mechanism for gene therapy components. Vectors are deactivated viruses that have been changed in a lab to prevent them from multiplying or causing an illness.
If someone has previously been exposed to a virus that is similar to a gene therapy vector, antibodies will be waiting to attack it. This will prevent the gene therapy from working and could cause harmful side effects. This is why antibody testing is part of the eligibility process; if levels are too high, treatment may not be an option.

Next up: Getting ready for treatment
Now that you’ve seen who may be eligible for ELEVIDYS, learn more about what to expect before the infusion.
